«The Unseen World: Introduction to Rodent Dentition Microscopy»
«Why Microscopic Analysis Matters»
Microscopic examination reveals enamel layering, dentin tubule orientation, and incisor wear patterns that are invisible to the naked eye. These details enable precise measurement of tooth growth rates and identification of microfractures that precede clinical failure.
The technique supports several scientific objectives:
- Quantification of mineral density variations across the crown and root, informing models of mechanical resilience.
- Detection of bacterial colonization and early-stage lesions, allowing timely intervention in laboratory animal health programs.
- Comparison of dental morphology among rodent species, contributing to phylogenetic analyses and evolutionary inference.
- Evaluation of the effects of dietary compounds or pharmacological agents on tooth development, guiding toxicological assessments.
Data acquired at high magnification also improve the calibration of imaging software used in dental research, ensuring consistency across studies. By linking microscopic features to functional outcomes, researchers obtain a reliable basis for hypothesis testing and risk assessment.
Overall, microscopic analysis transforms visual records of rodent dentition into quantitative evidence, strengthening the validity of experimental conclusions and enhancing reproducibility.
«Tools and Techniques for Capturing Mouse Teeth Photos»
Capturing high‑resolution images of mouse dentition requires specialized equipment and precise procedures. The combination of magnification, illumination, and image acquisition tools determines the clarity and reproducibility of the results.
- Stereomicroscope with interchangeable objectives (4×–40×) for variable magnification.
- DSLR or mirrorless camera equipped with a macro adapter to achieve 1:1 reproduction.
- Ring flash or fiber‑optic light source providing uniform, shadow‑free illumination.
- Motorized focus‑stacking rail to automate incremental focus steps and reduce manual error.
- Calibration slide or micrometer for scale verification and measurement accuracy.
Sample preparation techniques enhance detail visibility and protect specimen integrity:
- Euthanize and dissect the mouse head using aseptic instruments.
- Rinse the oral cavity with isotonic saline to remove debris.
- Apply a thin layer of glycerol or silicone oil to reduce surface reflections.
- Secure the mandible or maxilla on a non‑reflective platform with minimal pressure to avoid tooth displacement.
Imaging workflow optimizes data quality:
- Align the microscope and camera axis to ensure the optical center coincides with the tooth surface.
- Set exposure parameters (ISO, shutter speed, aperture) to balance depth of field and signal‑to‑noise ratio.
- Execute focus stacking, capturing a series of images at 0.1 mm depth intervals.
- Merge the stack using dedicated software (e.g., Helicon Focus) to produce a composite with extended depth of field.
- Apply contrast enhancement and background subtraction only when necessary for analysis, preserving raw data for quantitative measurements.
Consistent documentation of equipment settings, specimen orientation, and calibration references enables reproducibility across experiments and facilitates comparative studies of rodent dental morphology.
«Anatomy of Rodent Teeth Under the Microscope»
«Incisors: The Cutting Edge»
«Enamel and Dentin Structure»
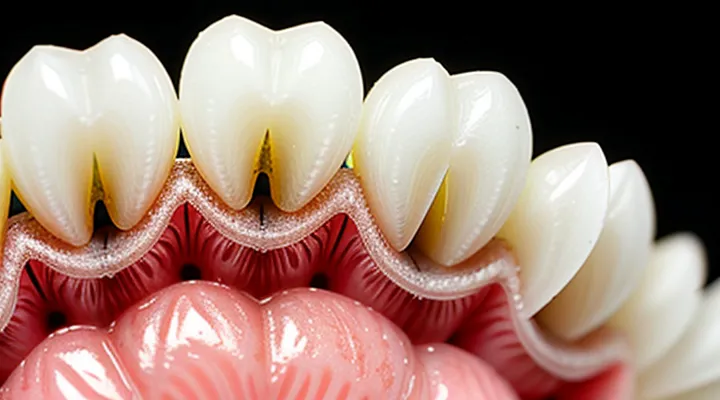
The enamel covering a mouse’s incisors forms the outermost, highly mineralized layer. It consists predominantly of hydroxyapatite crystals organized into tightly packed prisms that run perpendicular to the tooth surface. This arrangement creates a hard, wear‑resistant barrier while allowing the underlying tissue to be exposed during continuous growth.
Beneath enamel lies dentin, a less mineralized matrix composed of collagen fibers, dentinal tubules, and a lower concentration of hydroxyapatite. The tubules extend from the pulp chamber toward the enamel‑dentin junction, providing pathways for nutrient transport and sensory signaling. Dentin’s composition grants flexibility, enabling the tooth to resist fracture despite the high forces generated during gnawing.
Key structural features observable in high‑magnification imaging include:
- Enamel prisms with distinct orientation patterns that differ between the labial and lingual surfaces.
- The enamel‑dentin junction (EDJ), a transitional zone where enamel crystals interlock with dentin, enhancing mechanical cohesion.
- Dentinal tubules arranged radially, varying in diameter from the pulp outward, which influence permeability and hardness gradients.
The enamel’s thickness on mouse incisors exceeds that of molars, reflecting the need for persistent self‑sharpening. Dentin thickness remains relatively constant, supporting the rapid deposition of enamel at the growth front. Together, these layers maintain a functional tooth capable of continuous eruption and efficient processing of food.
«Continuous Growth Mechanism»
Microscopic examination of mouse dentition reveals a specialized system that compensates for constant enamel wear. The incisors grow throughout the animal’s life, a process driven by a stem‑cell niche located at the apical end of each tooth.
At the base of the incisor, proliferating epithelial and mesenchymal cells generate new enamel and dentin. These cells divide rapidly, migrate outward, and differentiate into ameloblasts and odontoblasts, which deposit mineralized matrix. The rate of cell division matches the rate of material loss at the occlusal surface, ensuring a stable length.
Key elements of the continuous growth mechanism include:
- Stem‑cell reservoir: Located in the cervical loop, provides a constant supply of progenitor cells.
- Differentiation gradient: Spatial cues direct cells to become enamel‑forming or dentin‑forming lineages.
- Matrix secretion: Ameloblasts lay down enamel ribbons; odontoblasts lay down dentin beneath.
- Self‑regulating feedback: Mechanical wear signals adjust proliferative activity to maintain equilibrium.
The system operates without external hormonal triggers, relying on intrinsic genetic programs and local signaling pathways such as Wnt, BMP, and Shh. Disruption of any component leads to abnormal tooth length, highlighting the precision of this self‑sustaining growth process.
«Molars: Grinding and Processing»
«Cusps and Ridges: Variations Across Species»
Microscopic imaging of rodent dentition reveals distinct patterns in cusp and ridge architecture that differentiate species. In mice, the molar surface displays a limited number of low, blunt cusps connected by shallow enamel ridges, whereas the incisor exhibits a single, continuously growing edge without discrete cusps.
Variations across common laboratory and wild rodents include:
- House mouse (Mus musculus): three modest cusps on the first molar; ridges run parallel to the occlusal plane, providing limited shearing ability.
- Deer mouse (Peromyscus maniculatus): four pronounced cusps with sharper apexes; ridges form a pronounced V‑shaped pattern that enhances grinding of seeds.
- Vole (Microtus spp.): five tightly packed cusps; ridges interlock, creating a complex mosaic suited for fibrous plant material.
- Hamster (Mesocricetus auratus): two broad cusps separated by a deep central ridge; the arrangement supports gnawing of hard kernels.
- Beaver (Castor canadensis): extensive series of elongated ridges rather than distinct cusps; the design maximizes abrasive wear on woody tissue.
These morphological differences correspond to dietary specializations. Species that consume primarily seeds and grains develop sharper cusps and intersecting ridges to facilitate crushing, while those that process fibrous or woody matter rely on elongated ridges that increase surface area for abrasion.
Accurate documentation of cusp‑ridge patterns through high‑resolution microscopy informs comparative dental biology, aids in species identification, and supports investigations into evolutionary adaptations of feeding mechanics.
«Wear Patterns and Diet Indicators»
Microscopic examination of mouse dentition reveals surface alterations that directly reflect masticatory behavior and nutritional intake. Enamel loss, cusp flattening, and striation depth serve as measurable indicators of mechanical stress imposed by specific food items.
Wear patterns exhibit distinct characteristics:
- Uniform abrasion across occlusal surfaces suggests a diet dominated by soft, homogeneous matter such as grains or laboratory chow.
- Localized pitting and deep scratches correspond to ingestion of fibrous or abrasive components, including seed hulls or coarse plant material.
- Asymmetrical wear on one side of the incisors indicates preferential chewing direction, often linked to the animal’s handling technique for irregularly shaped food.
Analysis of dentinal exposure provides further insight. Increased exposure of dentin layers correlates with prolonged consumption of high‑fiber diets, which accelerate enamel attrition. Conversely, limited dentin visibility aligns with low‑abrasion feeding regimes, reflecting a nutrient profile rich in easily digestible substances.
Quantitative assessment of wear depth, measured in micrometres, enables comparison across experimental groups. Consistent differences in wear magnitude can be attributed to variations in diet composition, allowing researchers to infer dietary preferences and nutritional adequacy without direct observation of feeding behavior.
«Common Dental Anomalies and Diseases»
«Malocclusion: Misalignment Issues»
Microscopic imaging of rodent dentition reveals frequent occurrence of malocclusion, a condition where upper and lower incisors fail to meet in a functional plane. The high‑resolution view exposes wear patterns, crown angles, and alveolar bone adaptation that indicate misalignment.
Key characteristics of rodent malocclusion include:
- Over‑growth of incisors: continuous eruption outpaces wear, producing elongated crowns that extend beyond the occlusal plane.
- Cross‑bite: lateral displacement of one or more incisors, often visible as asymmetrical enamel exposure.
- Open‑bite: failure of the incisor tips to contact, resulting in a vertical gap observable in the sagittal section.
- Rotational displacement: twisting of the crown around its long axis, detectable by irregular enamel bands.
Primary etiological factors are:
- Genetic predisposition – allelic variations affecting mandibular development and tooth eruption timing.
- Dietary texture – soft or processed foods reduce natural abrasion, allowing excessive growth.
- Mechanical trauma – injuries to the jaw or teeth disrupt normal occlusal alignment.
- Neuromuscular disorders – impaired masticatory muscle function alters bite forces.
Consequences evident in microscopic sections include:
- Enamel fractures caused by abnormal stress concentrations.
- Periodontal bone remodeling where alveolar sockets expand to accommodate misaligned crowns.
- Altered gnawing efficiency, leading to reduced food intake and weight loss.
Diagnostic protocol based on microscopic evaluation involves:
- Measuring incisor length from root apex to tip; a length exceeding 2 mm beyond the occlusal plane signals over‑growth.
- Calculating the angle between upper and lower incisor axes; deviation greater than 10° indicates cross‑bite.
- Assessing enamel thickness uniformity; irregularities suggest rotational displacement.
Management strategies derived from the observed morphology are:
- Regular trimming of incisors to restore proper length and angle, performed under magnification to avoid pulp exposure.
- Diet modification incorporating coarse, fibrous materials that promote natural wear.
- Genetic screening for breeding colonies to reduce hereditary incidence.
- Therapeutic appliances such as custom‑fit bite plates that guide proper occlusion during growth phases.
The microscopic perspective provides precise criteria for identifying and correcting misalignment, enabling effective prevention of secondary dental pathology in laboratory and pet rodent populations.
«Caries: The Formation of Cavities»
Microscopic imaging of mouse incisors reveals the early stages of dental caries, allowing precise observation of cavity formation at the cellular level. The high‑resolution view captures enamel surface irregularities where bacterial colonies initiate acid attacks.
Caries development follows a defined sequence:
- Bacterial fermentation of dietary carbohydrates produces organic acids.
- Acids lower local pH, triggering mineral dissolution from enamel crystals.
- Demineralized zones expand as hydroxyapatite loss exceeds remineralization.
- Advanced lesions breach the enamel–dentin junction, creating a cavity cavity.
Rodent incisors differ from human teeth by continuous growth and a gradient of enamel thickness, which influences lesion distribution. Enamel is thicker on the labial surface, providing greater resistance, while the lingual side exhibits thinner enamel and higher susceptibility to acid erosion. Microscopic sections show preferential demineralization on the lingual aspect, correlating with the pattern of bacterial colonization observed in the images.
Understanding cavity formation in this model supports the evaluation of preventive agents, such as fluoride compounds and probiotic strains, by measuring changes in mineral loss rates. The detailed visual data serve as a benchmark for assessing therapeutic efficacy and for extrapolating findings to human oral health research.
«Periodontal Disease: Gum and Bone Health»
Microscopic imaging of rodent dentition reveals the structural relationship between the enamel surface, periodontal ligament, gingival tissue, and alveolar bone. In rodents, the continuous growth of incisors demands a dynamic balance of tissue remodeling; any disruption manifests as periodontal disease, characterized by inflammation of the gum and resorption of supporting bone.
Key pathological features observable at high magnification include:
- Infiltration of neutrophils and macrophages into the gingival sulcus, indicating acute inflammatory response.
- Thickening of the junctional epithelium and loss of collagen fibers within the periodontal ligament, reducing attachment strength.
- Erosion of the alveolar crest and formation of resorption lacunae, visible as irregular depressions on the bone surface.
- Presence of bacterial biofilm on the enamel and interproximal areas, serving as a persistent source of toxin release.
These microscopic signs correspond to clinical outcomes such as gum recession, increased pocket depth, and tooth mobility. Early detection in rodent models, facilitated by high‑resolution photographs, allows quantitative assessment of tissue loss and evaluation of therapeutic interventions targeting inflammatory pathways and bone regeneration.
Maintaining gingival health in such models requires regular monitoring of plaque accumulation, controlled diet to limit abrasive wear, and administration of anti‑inflammatory agents when histological analysis indicates elevated cytokine expression. The integration of detailed visual data with histopathological scoring provides a robust framework for studying the mechanisms that drive gum and bone degradation and for testing strategies aimed at preserving periodontal integrity.
«Tumors and Cysts: Rare Pathologies»
Microscopic imaging of mouse dentition reveals occasional neoplastic and cystic lesions that differ markedly from common dental abnormalities. These rare pathologies affect the enamel‑forming ameloblasts, dentin‑producing odontoblasts, or the supporting periodontal tissues, often altering the architecture visible at high magnification.
Observed tumor types include:
- Ameloblastic carcinoma: aggressive epithelial tumor arising from ameloblasts, characterized by cellular pleomorphism and invasive growth into surrounding bone.
- Odontogenic fibroma: benign mesenchymal proliferation with sparse epithelial islands, producing a fibrous stroma that may compress adjacent dentin.
- Cementoblastoma: neoplasm of cementoblasts forming mineralized masses attached to root surfaces, detectable as irregular radiopaque structures in micrographs.
- Squamous cell carcinoma of the oral mucosa: epithelial malignancy extending into the gingival margin, occasionally involving the tooth‑supporting apparatus.
Cystic formations identified in rodent dental sections comprise:
- Odontogenic keratocyst: thin‑walled cavity lined by keratinizing epithelium, often situated in the mandibular body adjacent to developing molars.
- Radicular cyst: inflammatory lesion surrounding the apex of a non‑vital tooth, presenting as a fluid‑filled sac with a fibrous capsule.
- Dentigerous cyst: expansion of the follicular space surrounding an unerupted tooth germ, evident as a clear lumen encircling the crown.
Histopathological assessment relies on hematoxylin‑eosin staining, immunohistochemical markers (e.g., Ki‑67, p53) for proliferative activity, and electron microscopy to confirm ultrastructural features. Early detection in microscopic slides aids in distinguishing these uncommon entities from routine dental wear or infection, thereby informing experimental models of oral pathology and therapeutic interventions.
«Comparative Microscopy: Mouse vs. Other Rodents»
«Size and Shape Variations»
Microscopic imaging of mouse dentition reveals pronounced variability in both dimensions and morphology of the incisors and molars. The enamel‑covered incisors exhibit a longitudinal gradient: the anterior portion averages 1.8 mm in length, while the posterior segment shortens to approximately 1.2 mm. Cross‑sectional measurements show a consistent oval shape, with the major axis ranging from 0.35 mm to 0.45 mm and the minor axis from 0.20 mm to 0.28 mm. This elongation aligns with the functional demand for continuous gnawing.
The cheek teeth display distinct size classes among individuals:
- First molar (M1): length 0.95 mm ± 0.07, width 0.70 mm ± 0.05; crown shape typically triangular with rounded apex.
- Second molar (M2): length 0.85 mm ± 0.06, width 0.65 mm ± 0.04; crown shape often trapezoidal, exhibiting a slight posterior taper.
- Third molar (M3, when present): length 0.70 mm ± 0.05, width 0.55 mm ± 0.03; crown shape varies from rectangular to irregular, reflecting developmental plasticity.
Shape analysis via scanning electron microscopy identifies two recurring patterns in incisor cross‑sections: a classic “D‑shaped” profile with a flat dorsal surface and a “U‑shaped” profile where the dorsal surface curves gently. The proportion of each profile correlates with genetic lineages, suggesting heritable determinants.
Variations in enamel thickness further differentiate tooth morphology. Incisal enamel averages 12 µm on the labial side, tapering to 6 µm on the lingual side. Molar enamel thickness ranges from 8 µm to 14 µm, with the highest values located at cusp tips. These measurements influence wear resistance and are critical for interpreting dietary adaptations in laboratory mouse strains.
«Dietary Adaptations Reflected in Tooth Morphology»
The microscopic examination of rodent dentition reveals precise correlations between tooth architecture and nutritional intake. Incisors display continuous growth, a trait that accommodates the abrasive nature of seeds and fibrous plant material. Enamel thickness varies along the labial surface, reinforcing regions that encounter frequent gnawing forces.
Molar cusps exhibit distinct morphologies that correspond to preferred food categories. Sharp, pointed cusps dominate in species consuming insects or soft tissues, facilitating penetration and shearing. Flattened, ridged surfaces appear in granivorous rodents, enhancing grinding efficiency for hard kernels.
- High‑crowned (hypsodont) molars for processing gritty, mineral‑rich diets.
- Low‑crowned (brachydont) molars in omnivorous species that ingest softer matter.
- Pronounced enamel ridges on incisors to resist wear from persistent gnawing.
- Reduced enamel on occlusal surfaces where rapid wear accelerates tooth renewal.
These morphological adaptations enable rodents to exploit diverse ecological niches, linking dental form directly to dietary strategy. Understanding the relationship between feeding behavior and tooth structure informs comparative anatomy, evolutionary biology, and pest management practices.
«Future Directions in Rodent Dental Research»
«Advances in Imaging Technology»
Recent developments in high‑resolution imaging have reshaped the study of rodent incisor morphology. Enhanced detector sensitivity and automated image acquisition now permit sub‑micron detail without extensive specimen preparation.
Key technologies include:
- Scanning electron microscopy (SEM) with low‑vacuum chambers, delivering surface topography and elemental contrast at nanometer scales.
- Confocal laser scanning microscopy (CLSM), providing optical sectioning of enamel and dentin layers while preserving three‑dimensional context.
- Synchrotron‑based micro‑computed tomography (micro‑CT), generating isotropic voxel sizes below 1 µm and enabling quantitative volumetric analysis of enamel thickness.
- Super‑resolution fluorescence microscopy, allowing visualization of protein distribution within developing tooth buds at molecular resolution.
These tools improve measurement accuracy of cusp geometry, enamel‑dentin junction curvature, and wear patterns. Integrated workflows combine automated stitching algorithms with machine‑learning segmentation, reducing manual annotation time and increasing reproducibility across laboratories. The resulting datasets support comparative studies of genetic knockouts, dietary effects, and evolutionary adaptations in murine models.
«Applications in Evolutionary Biology»
Microscopic imaging of mouse dentition provides precise morphological data that can be directly compared across extinct and extant species. By quantifying enamel thickness, cusp patterns, and root architecture, researchers reconstruct phylogenetic relationships and test hypotheses about lineage divergence.
Key evolutionary applications include:
- Morphometric analyses: High‑resolution measurements feed into multivariate statistics, revealing trait convergence or divergence among mammalian clades.
- Developmental timing: Tooth eruption sequences inferred from microscopic sections calibrate molecular clocks, improving estimates of divergence dates.
- Adaptive inference: Correlations between dental wear patterns and diet allow inference of ecological niches occupied by ancestral rodents.
- Genotype‑phenotype mapping: Detailed dental phenotypes serve as quantitative traits for genome‑wide association studies, linking genetic variation to evolutionary adaptations.
Integration of these data with fossil records refines models of mammalian radiation, clarifies the role of dental specialization in niche exploitation, and supports robust reconstructions of evolutionary pathways.