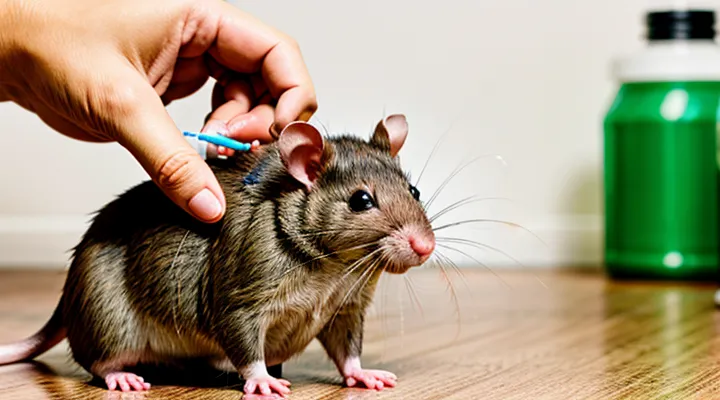
Introduction to Intramuscular Injections in Rats
Purpose and Importance
The purpose of administering an intramuscular injection to a laboratory rat is to introduce a substance directly into the muscle tissue, where it can be absorbed into the systemic circulation more rapidly than subcutaneous routes. This method enables precise dosing, reliable pharmacokinetic profiling, and targeted delivery of agents that require muscular uptake.
The significance of executing the procedure correctly includes several measurable outcomes:
- Consistent absorption rates that reduce inter‑subject variability.
- Minimized tissue trauma, which lowers the risk of inflammation and infection.
- Decreased animal discomfort, supporting compliance with welfare regulations.
- Enhanced reproducibility of experimental results, facilitating comparison across studies.
- Alignment with institutional and regulatory standards for humane animal research.
Accurate technique therefore underpins data integrity, animal health, and ethical responsibility in biomedical investigations involving rodents.
Ethical Considerations and Animal Welfare
Ethical justification for administering intramuscular injections to rats requires clear scientific purpose, compliance with institutional and national regulations, and demonstration that no alternative method can achieve the same objectives. Researchers must obtain approval from an Animal Care and Use Committee, providing detailed protocols that specify the number of animals, justification for the procedure, and anticipated benefits.
Animal welfare considerations focus on minimizing pain, distress, and long‑term effects. Key practices include:
- Using the smallest effective needle gauge and volume to reduce tissue trauma.
- Applying appropriate local anesthetic or analgesic agents before and after injection.
- Conducting injections under brief restraint or light anesthesia to prevent struggling and associated stress.
- Monitoring animals for immediate adverse reactions and for delayed signs of discomfort, with criteria for humane intervention.
Refinement strategies extend to training personnel in precise injection techniques, ensuring consistent landmark identification, and employing aseptic methods to prevent infection. Documentation of each injection—site, depth, angle, and volume—supports reproducibility and accountability.
The principle of reduction mandates that the experimental design incorporate statistical power analyses to avoid unnecessary animal use. When possible, combine multiple procedures within a single session to limit handling frequency. Continuous evaluation of welfare outcomes informs protocol adjustments, reinforcing a responsible approach to intramuscular administration in rodent research.
Pre-Injection Preparation
Equipment and Supplies
Needles and Syringes
Needles and syringes are the primary instruments for delivering intramuscular medication to laboratory rats. Selecting appropriate dimensions minimizes tissue trauma and ensures accurate dosing.
- Gauge: 25‑27 G needles provide sufficient rigidity for penetration of the quadriceps femoris while limiting muscle fiber disruption.
- Length: 5‑7 mm shafts reach the muscle bulk without contacting bone in adult rats weighing 200‑300 g.
- Volume: 0.5‑1 mL syringes accommodate typical injection volumes (0.1‑0.3 mL) while allowing precise measurement.
Sterile, single‑use syringes prevent cross‑contamination. Prior to injection, remove the needle cap, verify the absence of cracks, and confirm the syringe plunger moves smoothly. Attach the needle at a 90° angle to the skin, insert to the full length, and aspirate briefly to rule out vascular entry. Deliver the dose steadily, then withdraw the needle and apply gentle pressure with a sterile gauze pad to reduce bleeding.
Proper storage of needles and syringes includes sealed packaging at room temperature, protected from moisture. Discard used devices in a designated sharps container immediately after the procedure.
Medications and Solutions
Intramuscular administration in rats requires careful selection of pharmacological agents and carrier solutions to ensure accurate dosing, tissue compatibility, and reproducible results.
Commonly used medication categories include:
- Anesthetics – ketamine, xylazine, and combination formulations; concentrations adjusted to 10–20 mg ml⁻¹ for 0.1 ml injections in adult rodents.
- Analgesics – buprenorphine (0.05–0.1 mg ml⁻¹) and meloxicam (1 mg ml⁻¹); prepared in sterile saline to avoid precipitation.
- Antibiotics – enrofloxacin (2 mg ml⁻¹) and gentamicin (5 mg ml⁻¹); dissolved in isotonic solutions to maintain osmolarity.
- Hormones and growth factors – insulin, recombinant mouse erythropoietin; filtered through 0.22 µm membranes to remove particulates.
Carrier solutions must meet specific criteria:
- Isotonicity – 0.9 % sodium chloride or lactated Ringer’s solution to prevent cellular edema.
- pH stability – adjusted to 7.2–7.4 using sterile buffers (HEPES or phosphate) to preserve drug activity.
- Sterility – filtered and autoclaved where compatible; aseptic technique mandatory during preparation.
Key preparation steps:
- Verify drug solubility; if insoluble, employ co‑solvents (e.g., DMSO ≤ 5 % v/v) or formulate as a suspension with sterile oil (e.g., sesame oil).
- Perform a visual inspection for precipitates or color change; discard any compromised solution.
- Label each vial with concentration, volume, expiration time, and storage temperature; many agents require refrigeration at 2–8 °C and protection from light.
Stability considerations:
- Small‑molecule drugs typically remain stable for 24 h at room temperature; longer storage necessitates refrigeration.
- Peptide and protein solutions often degrade within hours; add protease inhibitors and store on ice during use.
Dosing accuracy depends on precise volume measurement; calibrated syringes (0.1 ml capacity) provide repeatable delivery. The injection site (commonly the quadriceps or gluteus) should be free of hair and disinfected with 70 % ethanol before needle insertion.
Overall, adherence to these medication and solution guidelines supports reliable intramuscular delivery in rat models, minimizes adverse tissue reactions, and enhances reproducibility across experimental protocols.
Restraint Devices
Restraint devices are indispensable for delivering intramuscular injections to rats with precision and minimal distress. Proper immobilization aligns the target muscle, stabilizes the animal, and protects the operator from accidental needle injury.
Common devices include:
- Manual restrainers that hold the rat’s torso while exposing the hind limb.
- Transparent tube restrainers that limit movement and provide visual access.
- Plexiglass or acrylic chambers equipped with adjustable nose and tail slots.
- Anesthetic induction chambers used when sedation is required before injection.
Selection criteria focus on compatibility with the animal’s size, the injection site, and the duration of the procedure. The device must allow clear visualization of the quadriceps or gluteal region, permit gentle limb extension, and enable rapid release in emergencies. Ergonomic design reduces operator fatigue and decreases handling time.
Effective use follows a defined sequence:
- Inspect the device for cracks, sharp edges, or loose components.
- Place the rat gently into the restrainer, ensuring the head remains upright and the hind limb is accessible.
- Secure the torso without excessive pressure; adjust the openings to prevent escape.
- Extend the target limb, verify the injection site is palpable and free of hair or debris.
- Perform the injection using the recommended angle and depth.
- Release the animal promptly, monitor for signs of distress, and return it to its housing.
Maintenance protocols require thorough cleaning after each use, disinfection with an appropriate veterinary sanitizer, and routine verification of structural integrity. Replace any worn or damaged parts immediately to preserve sterility and functionality.
Safety measures include wearing needle‑proof gloves, keeping a sharps container within arm’s reach, and establishing a clear plan for rapid animal release if resistance escalates. Regular training reinforces correct handling techniques and upholds the standards of intramuscular administration in rodent research.
Animal Preparation
Anesthesia and Analgesia
Anesthesia and analgesia are essential components of safe intramuscular administration in laboratory rats. Selection of an anesthetic regimen should consider rapid onset, predictable depth, and minimal interference with muscle tone. Injectable agents such as ketamine (50–100 mg/kg) combined with xylazine (5–10 mg/kg) provide reliable sedation and muscle relaxation, allowing accurate needle placement. Inhalational options, including isoflurane delivered at 1–3 % in oxygen, enable rapid adjustment of anesthetic depth and quick recovery after the procedure.
Analgesic coverage must extend before, during, and after injection to prevent nociceptive responses. A pre‑emptive dose of buprenorphine (0.05 mg/kg, subcutaneously) administered 15–30 minutes prior to needle insertion reduces acute pain. For procedures expected to cause prolonged discomfort, repeat dosing every 8–12 hours maintains effective analgesia. Non‑steroidal anti‑inflammatory drugs (e.g., meloxicam 1–2 mg/kg, subcutaneously) can be added to address inflammatory pain without compromising sedation.
Key considerations for anesthesia and analgesia in this context include:
- Drug selection that preserves muscle tone sufficient for injection accuracy.
- Dosage calculations based on individual body weight to avoid overdose.
- Monitoring of respiratory rate, heart rate, and reflexes throughout the procedure.
- Immediate availability of reversal agents (e.g., atipamezole for xylazine) if excessive sedation occurs.
- Documentation of analgesic timing relative to injection to ensure continuous pain control.
Site Preparation and Asepsis
Proper intramuscular delivery in rats demands a sterile injection site. The procedure begins with secure restraint to prevent movement. Hair over the chosen muscle—typically the quadriceps or gluteus—is clipped close to the skin using sterile shears. Immediately after clipping, apply a broad‑spectrum antiseptic (e.g., 70 % isopropanol or chlorhexidine) with a sterile gauze pad, covering the entire area. Allow the antiseptic to air‑dry before proceeding; residual moisture can dilute the injectable solution and compromise sterility.
Key steps for site preparation and asepsis:
- Clip fur to expose skin without damaging underlying tissue.
- Disinfect with a single‑use applicator soaked in an approved antiseptic.
- Perform a gentle circular motion for 10–15 seconds to ensure uniform coverage.
- Let the area dry completely; do not blot or wipe with a dry cloth.
- Inspect the site for visible contaminants; repeat disinfection if any residue remains.
Maintaining a sterile field throughout the injection minimizes the risk of infection and ensures reproducible pharmacological outcomes.
Injection Technique
Restraint Methods
Manual Restraint
Manual restraint provides the stability required to deliver an intramuscular dose accurately while limiting the animal’s movement. Proper handling reduces the risk of needle misplacement and prevents tissue damage.
Before restraint, verify that the injection site is exposed, the syringe is prepared, and the work area is free of obstacles. Allow the rat to acclimate to the surface for a brief period to diminish stress responses.
- Grasp the animal by the base of the tail with the thumb and forefinger of the dominant hand.
- Apply gentle pressure to the dorsal thorax with the palm of the same hand, securing the forelimbs against the body.
- Use the opposite hand to hold the hindquarters, keeping the hind limbs flexed and the abdomen slightly lifted.
- Position the rat’s hind leg so that the quadriceps muscle is accessible; the leg should be extended but not hyperextended.
- Maintain the grip for the duration of needle insertion, then release promptly after the injection is complete.
Apply steady, modest force; excessive pressure can cause bruising or fractures. Keep the animal’s head at a neutral angle to avoid airway obstruction. Monitor for signs of struggle; if resistance increases, pause and reassess the hold before proceeding.
Operators should wear protective gloves, use puncture‑resistant containers for sharps, and maintain a clear line of sight to the injection site throughout the procedure. Regular training reinforces consistent technique and enhances both animal welfare and experimental reliability.
Chemical Restraint
Chemical restraint is a prerequisite for safe intramuscular administration in laboratory rats. The agent must produce rapid, reversible immobilization without compromising physiological parameters that could affect experimental outcomes. Commonly employed drugs include a combination of ketamine and xylazine, medetomidine, or isoflurane induction followed by brief intramuscular delivery of a sedative cocktail.
Prior to injection, verify the animal’s weight, calculate the exact dose, and dilute the drug to a volume not exceeding 0.1 mL per 100 g body weight. Use a sterile 1‑mL syringe equipped with a 27‑ to 30‑gauge needle; the needle length should reach the muscle without penetrating the bone (typically 5–8 mm for adult rats). The injection site is the lateral thigh (quadriceps) or the dorsal lumbar region, where the muscle mass is substantial and the risk of nerve injury is minimal.
Procedure:
- Restrain the rat in a transparent tube or a specialized restrainer that allows access to the injection site while maintaining the animal’s comfort.
- Clean the skin with 70 % ethanol; allow it to dry to reduce contamination.
- Insert the needle at a 90° angle, advance until a slight resistance is felt, indicating entry into the muscle.
- Depress the plunger smoothly; avoid rapid delivery that could cause tissue trauma.
- Withdraw the needle promptly, apply gentle pressure with sterile gauze to minimize bleeding.
Post‑injection monitoring includes observation of respiratory rate, heart rate, and reflexes until the animal regains full mobility. Record the drug batch, dose, and any adverse reactions in the protocol log. Proper execution of chemical restraint ensures consistent drug distribution, reduces stress‑induced variability, and supports reproducible experimental results.
Choosing the Injection Site
Thigh Muscles
The thigh region of the rat provides a reliable site for delivering intramuscular injections because the muscle mass is sufficient to accommodate the volume of most experimental preparations while minimizing the risk of injury to adjacent structures.
The primary muscles targeted are the vastus lateralis and the quadriceps femoris group. The vastus lateralis lies on the lateral aspect of the thigh and is easily identified by palpation of the bony ridge extending from the greater trochanter to the knee joint. The quadriceps femoris occupies the anterior surface and can be accessed by positioning the needle over the mid‑thigh, approximately one‑third of the distance from the hip to the knee.
Key anatomical considerations:
- Locate the lateral femoral ridge; insert the needle perpendicular to the skin surface at a point 0.5–1.0 cm distal to this ridge.
- Avoid the femoral nerve, which runs medially; maintain a lateral trajectory when possible.
- Use a needle length of 0.5–0.75 in (13–19 mm) for adult rats (250–300 g) to ensure penetration of the muscle without contacting bone.
- Aspirate gently before injection to confirm placement within the muscle tissue.
- Withdraw the needle at the same angle of entry to reduce tissue trauma.
Aseptic protocol includes cleaning the injection site with 70 % isopropyl alcohol, allowing it to dry, and using sterile gloves and equipment throughout the procedure. Proper restraint, such as a dorsal‑sacral hold, stabilizes the animal and reduces movement, contributing to accurate needle placement.
Adhering to these anatomical landmarks and technical parameters ensures consistent delivery of substances into the thigh musculature of laboratory rats while maintaining animal welfare and experimental integrity.
Gluteal Muscles
The gluteal region provides a reliable site for delivering intramuscular agents to laboratory rats. The primary muscle mass consists of the gluteus maximus, gluteus medius, and gluteus minimus, each covering a substantial portion of the posterior thigh. These muscles are well‑vascularized, allowing rapid systemic absorption of injected compounds.
Accurate placement depends on clear external landmarks. Palpate the greater trochanter and draw an imaginary line to the ischial tuberosity; the midpoint of this line marks the optimal injection zone. The injection should be performed on the right side to reduce variability linked to dominant limb use.
Key technical parameters:
- Needle gauge: 25‑27 G for volumes up to 0.5 mL; larger gauges for viscous solutions.
- Needle length: 5 mm for adult rats (250‑300 g); 3 mm for juveniles (<150 g).
- Insertion angle: 90° to the skin surface, ensuring perpendicular entry into muscle fibers.
- Depth: advance needle until the hub contacts the skin, then insert the full length of the needle; this places the tip within the muscle belly.
- Volume: do not exceed 0.1 mL per 100 g body weight to avoid tissue distension.
Procedural safeguards include confirming needle placement by gently aspirating before injection, using sterile technique to prevent infection, and observing the animal for at least five minutes post‑administration to detect adverse reactions. Proper restraint—using a tail‑squeeze or a dedicated restraining device—maintains animal stability while minimizing stress.
Administration Steps
Needle Insertion
Needle insertion determines the success of an intramuscular injection in rats. Precise placement minimizes tissue trauma and ensures reliable drug delivery.
- Identify the dorsal thigh (quadriceps) as the preferred site; the muscle is large, well‑vascularized, and accessible without excessive movement.
- Secure the animal in a restrainer or by gentle manual hold; maintain the limb in extension to expose the injection area.
- Choose a 25‑27 G needle, length 5–7 mm, appropriate for the animal’s size and the depth of the target muscle.
- Align the needle at a 90° angle to the skin surface; a perpendicular approach penetrates the subcutaneous layer and enters the muscle directly.
- Insert the needle smoothly until the hub contacts the skin, then advance the full length until resistance indicates entry into the muscle belly.
- Verify placement by a brief aspiration; absence of blood confirms intramuscular location.
- Deliver the prescribed volume steadily; avoid rapid injection that may cause reflux or tissue disruption.
- Withdraw the needle swiftly at the same 90° angle; apply gentle pressure with a sterile swab to the puncture site.
Consistent execution of these steps reduces variability in drug absorption and supports reproducible experimental outcomes.
Aspiration
Aspiration verifies that the needle has not entered a blood vessel before delivering the drug into the muscle. After the needle penetrates the selected site—commonly the quadriceps or gluteus—the syringe is held steady and the plunger is pulled back slightly. A brief pause of 1–2 seconds allows any intravascular blood to enter the barrel. The presence of blood indicates vascular puncture; the needle must be withdrawn and repositioned before proceeding.
Key points for reliable aspiration in rodents:
- Use a 1‑ml syringe with a 25‑ to 27‑gauge needle; larger gauges increase resistance and reduce the likelihood of blood draw.
- Insert the needle at a 90° angle to the skin to achieve full muscular depth.
- Pull the plunger back 0.1–0.2 ml (approximately 10–20 % of the syringe capacity) to create negative pressure.
- Observe the barrel for a flash of red fluid; absence of blood confirms proper placement.
- If blood appears, discard the syringe, select a new site, and repeat the aspiration step.
Following aspiration, inject the calculated volume slowly (no faster than 0.1 ml per second) to minimize tissue trauma. Maintain the needle in place for an additional 5–10 seconds after delivery to reduce reflux. Proper execution of aspiration safeguards against systemic exposure, improves dosing accuracy, and minimizes adverse effects in experimental rats.
Injection of Solution
Injecting a solution into the rat’s muscle requires precise control of volume, concentration, and sterility. Use a sterile syringe and a 25‑27‑gauge needle, length 0.5–0.75 in, to reach the bulk of the muscle without penetrating bone. Prior to injection, confirm that the solution is at room temperature, free of particulates, and correctly labeled.
Select the quadriceps femoris or the gluteal region as the injection site. Shave or disinfect the area with 70 % ethanol, allowing the skin to dry before needle insertion. Insert the needle at a 90° angle to the skin surface, advancing until the hub contacts the muscle fascia. Verify proper placement by gentle aspiration; the presence of blood indicates vascular entry and requires needle repositioning.
Deliver the solution steadily, typically not exceeding 0.1 mL per 100 g body weight to avoid tissue distension. After injection, withdraw the needle slowly, apply light pressure with sterile gauze, and observe the animal for at least five minutes for signs of distress or adverse reaction.
Key procedural steps:
- Prepare sterile equipment and solution.
- Choose appropriate muscle (quadriceps or gluteus).
- Disinfect injection site.
- Insert needle perpendicularly to muscle.
- Aspirate to confirm non‑vascular placement.
- Inject the prescribed volume at a controlled rate.
- Withdraw needle, apply gauze, monitor animal.
Adherence to these steps ensures reproducible delivery, minimizes tissue trauma, and maintains experimental integrity.
Needle Withdrawal
Needle withdrawal must be performed with a steady, controlled motion to prevent tissue trauma and drug leakage. After delivering the full dose, keep the needle in place for a brief pause (1–2 seconds) to allow the solution to disperse within the muscle fibers. Then withdraw the needle along the same trajectory used for insertion, maintaining the original angle (typically 90° to the skin surface) without tilting.
Key points for optimal withdrawal:
- Maintain a smooth, continuous pull; avoid jerking or rapid removal.
- Release the syringe plunger before extraction to eliminate negative pressure that could draw fluid back into the needle.
- Apply gentle pressure with a sterile gauze at the injection site immediately after removal to control minor bleeding and aid absorption.
- Discard the needle in a puncture‑proof container without recapping to reduce needlestick risk.
Consistent adherence to these actions minimizes muscle damage, reduces the likelihood of drug loss, and supports reproducible experimental outcomes in rodent studies.
Post-Injection Care and Monitoring
Observation for Adverse Reactions
Monitoring for adverse reactions after an intramuscular injection in rats is essential for validating technique and ensuring animal welfare. Observation begins immediately after needle removal and continues at regular intervals for at least 30 minutes, then at 2‑hour, 6‑hour, and 24‑hour checkpoints. Record each assessment in a dedicated log, noting time, animal identifier, and any deviations from normal behavior.
Key indicators of a negative response include:
- Local swelling or induration at the injection site
- Redness, heat, or discharge suggesting infection or tissue trauma
- Limping, altered gait, or inability to bear weight on the injected limb
- Excessive vocalization, agitation, or prolonged immobility
- Respiratory distress, tachypnea, or cyanosis
- Sudden loss of consciousness or seizure activity
If any sign appears, intervene promptly: apply a cold compress for swelling, administer an appropriate analgesic, and, when necessary, consult veterinary staff for antimicrobial therapy or supportive care. Document the intervention, dosage, and outcome.
Statistical analysis of adverse event frequency should be performed after a sufficient sample size is reached. Compare incidence rates across different needle gauges, injection volumes, and anatomical sites to identify procedural factors that increase risk. Adjust the injection protocol based on these findings to minimize future complications.
Pain Management
Effective pain control is a prerequisite for any intramuscular administration in laboratory rats. Analgesic protocols must be selected to match the procedure’s invasiveness, the animal’s physiological status, and the study’s ethical requirements.
- Pre‑procedure analgesia: administer a non‑steroidal anti‑inflammatory drug (e.g., meloxicam 1–2 mg kg⁻¹ subcutaneously) 30 minutes before injection; consider a short‑acting opioid (e.g., buprenorphine 0.05 mg kg⁻¹ intraperitoneally) for procedures expected to cause moderate tissue trauma.
- Local anesthesia: infiltrate the injection site with 0.5 % lidocaine (0.1 ml per 100 g body weight) to blunt nociceptive input during needle penetration.
- Sedation (optional): use a low‑dose combination of ketamine (10 mg kg⁻¹) and xylazine (2 mg kg⁻¹) intraperitoneally when handling stress is anticipated to exacerbate pain perception.
Post‑procedure monitoring includes observation of gait, weight bearing, and facial expression scores at 15‑minute intervals for the first hour and at 24‑hour checkpoints. Record analgesic administration times, dosages, and any adverse reactions in the animal’s health log. Adjust subsequent dosing based on observed pain indicators to maintain consistent comfort throughout the study.
Record Keeping
Accurate documentation is essential for reproducible intramuscular administration in rats. Each injection must be linked to a unique animal identifier, recording species, strain, age, weight, and housing conditions. The injection site (e.g., hindlimb, quadriceps) and anatomical landmarks should be noted to prevent repeated puncture of the same location.
Key data elements include:
- Date and exact time of administration
- Name of the operator performing the injection
- Drug name, concentration, and total volume delivered
- Needle gauge, length, and manufacturer
- Preparation details such as solvent, pH, and sterility checks
Observations made before, during, and after the procedure must be entered promptly. Pre‑injection signs (e.g., stress, grooming) and post‑injection responses (e.g., swelling, pain, behavioral changes) are critical for assessing technique efficacy and animal welfare.
Records should be stored in a system that ensures permanent, searchable access and complies with institutional and regulatory requirements. Digital databases allow for automated backup, audit trails, and cross‑referencing with other experimental variables. When paper forms are used, they must be archived in a secure, climate‑controlled environment and later transferred to an electronic system.
Regular review of the documentation identifies trends such as needle‑related complications or dosing errors, enabling corrective actions. Periodic audits verify that all entries are complete, legible, and conform to the standard operating procedure for intramuscular delivery in rodent models.